THE FORTNIGHTLY CLUB
Of
REDLANDS, CALIFORNIA
Founded 24 January 1895
Meeting Number 1784
4:00 P.M.
November 19, 2009
The Vitamin D Story
David Baylink, M.D.
(Presented as a part of a special discussion on Men’s Health with Fredric Rabinowitz, Ph.D. and Stan Korfmacher, M.D.)
Assembly Room, A. K. Smiley Public Library
What would you think if I told you that I knew how to decrease gastrointestinal cancers (for example, colon cancers) by more than 40%? What would you think if I told you that there was something out there that would reduce the development type 1 diabetes?
Vitamin D has been touted to do this and more. We are also talking about reducing the risk of heart attacks, strokes, periodontal disease, 18 different types of cancer and even decreasing all cause mortality. Too good to be true? May be; but what if these recent scientific studies are correct? Can you afford not to bet on this vitamin when the downside of taking adequate amounts of vitamin D is almost nonexistent? Besides, it's cheap.
In all began back in the winter of 1773 when a woman living near Manchester, England was forced to seek out the local apothecary because of excruciating limb pain. She called it "painful bones." At the end of the consultation, she was given a solution to rub on her painful limbs, once daily. From Here on the story is not entirely clear but apparently rubbing the solution on her limbs did not work; so she tried taking the solution by mouth. To her surprise, she began to experience some relief. Eventually, her pain scale probably went from about an 8 to 0. Once she became symptom-free she told her friends of her exciting discovery. Rumors of her success spread from neighbor to neighbor and eventually even the apothecary learned how to prescribe cod liver oil which is a good but yucky source of vitamin D.
After many years of research, we now know that vitamin D deficiency causes osteomalacia, a painful bone disease, which can be cured by either sunlight or oral vitamin D. We are a society so concerned about skin cancer and wrinkles that even in Southern California there are large numbers of elderly folk with vitamin D deficiency. I mentioned the elderly particularly because the efficiency of vitamin D production in the skin in response to sunlight is decreased in elderly. Which reminds me of my theme in this column and that is if you plan to get old be smart and practice preventative medicine, like taking adequate amounts of vitamin D.
To get adequate vitamin D you do not need to expose yourself to sunlight unless you wish to. However, in general, food is not a very good source of vitamin D and therefore we have to rely upon supplements unless you sunbathe and that might not even work if you're older. In general, the skin is very efficient in producing vitamin D upon sunlight light exposure.
However, this efficiency drops by more than 30% during aging. The government has recommended 600 IU per day of vitamin D for the elderly; whereas experts are now recommending that most elderly will require between 1000 and 2000 IU of vitamin D per day. Vitamin D by itself is now available in 1000 and 2000 IU tablets.
If you're stimulated by this column to review information yourself on the Internet about vitamin D and become convinced of its potential, you may want to establish how much you as an individual needs for optimal health. This is now simple to accomplish. Ask your family doctor for a vitamin D blood test (serum 25-hydroxy vitamin D). You should register at least 32 ng/ml, which is now considered to be the lower end of the normal range.
Another pertinent question is "can I get too much vitamin D"? The answer is yes, and if you did, you could develop too much calcium in the urine, which in turn could lead to kidney stones. However this complication will not occur if your serum level of 25-hydroxy vitamin D is 32 ng/ml or even modestly higher. Incidentally, if you have a history of kidney stones and want to be on the safe side, you could just drink more fluid because kidney stones develop when the urine is too concentrated.
I have emphasized the elderly because this group of citizens is at high risk for vitamin D deficiency. However, it is important to emphasize that vitamin D is important for everyone including infants. For example, breast fed infants are at risk for vitamin D deficiency because breast milk may contain relatively low amounts of vitamin D, depending on the vitamin D status of the mother. In a review of 394 publications on studies of vitamin D worldwide, the groups of individuals at risk for vitamin D deficiency were children, the elderly, and dark skin individuals; vitamin D production is less efficient in dark skinned individuals. Recently this type of information has been interpreted by experts to indicate that there is at global epidemic of vitamin D deficiency.
As I mentioned, my big picture theme for this column is preventative medicine. To this theme I would like to add one additional lifestyle suggestion to help prevent all kinds of infirmities. It's not a secret, its exercise, and like vitamin D, has no serious side effects when done appropriately. Vitamin D and exercise are the outdoor twins for better health. Do your exercise every day whether it's running or walking or bicycling or dancing. Be active and your body will respond favorably by producing strong bones, a strong heart and a clear mind.
*Portions of this section were presented in a newspaper column written by the author.
Does vitamin D improve brain function?**
“We know there are receptors for vitamin D throughout the central nervous system. In addition, animal and laboratory studies suggest vitamin D protects neurons and reduces inflammation.
Two new European studies looking at vitamin D and cognitive function have taken us one step further. The first study, led by neuroscientist David Llewellyn of the University of Cambridge, assessed vitamin D levels in more than 1,700 men and women from England, aged 65 or older. Subjects were divided into four groups based on vitamin D blood levels: severely deficient, deficient, insufficient (borderline) and optimum, then tested for cognitive function.
The scientists found that the lower the subjects’ vitamin D levels, the more negatively impacted was their performance on a battery of mental tests. Compared with people with optimum vitamin D levels, those in the lowest quartile were more than twice as likely to be cognitively impaired.
A second study, led by scientists at the University of Manchester in England and published online this past May, looked at vitamin D levels and cognitive performance in more than 3,100 men aged 40 to 79 in eight different countries across Europe. The data show that those people with lower vitamin D levels exhibited slower information-processing speed. This correlation was particularly strong among men older than 60 years.
Although we now know that low levels of vitamin D are associated with cognitive impairment, we do not know if high or optimum levels will lessen cognitive losses. It is also unclear if giving vitamin D to those who lack it will help them regain some of these high-level functions.
Because cognitive impairment is often a precursor for dementia and Alzheimer’s disease, vitamin D is a hot topic among Alzheimer’s scientists, who are racing to answer these questions. One scientist and, for example, is planning a study of vitamin D supplements in healthy, normal elderly adults living in an assisted-living community to see if it will affect their incidence of Alzheimer’s in the long term.
So how much is enough vitamin D? Experts say 1,000 to 2,000 IU daily—about the amount your body will synthesize from 15 to 30 minutes of sun exposure two to three times a week—is the ideal range for almost all healthy adults. Keep in mind, however, that skin color, where you live and how much skin you have exposed all affect how much vitamin D you can produce.
** From Scientific American, November 2009
Cinical Review
Prevention of Colorectal Cancer with Vitamin D
Dae S Rheem, M.D., Ph.D.,¹ David J Baylink, M.D., F.A.C.B.², Snorri Olafsson, M.D., Ph.D., M.P.H.,¹ Christian Jackson, M.D., ¹,³ Michael H. Walter, M.D., F.A.C.P., F.A.C.G.¹
¹Division of Gastroenterology, ²Department of Internal Medicine, Loma Linda University, Loma Linda, ³Jerry L. Pettis V. A. Medical Center, Loma Linda
The fact that colorectal cancer (CRC) is the second leading cause of cancer mortality in the United States emphasizes the need for more effective preventive and therapeutic modalities. There is growing evidence that vitamin D may reduce the incidence of CRC. Results of epidemiologic, in vitro, in vivo animal and clinical studies suggest that a low serum vitamin D level may be a serious risk factor for CRC and a high serum vitamin D level may reduce the risk for CRC. On a molecular level, vitamin D suppresses CRC development and growth by affecting cell proliferation, differentiation, apoptosis, and angiogenesis. Vitamin D insufficiency and CRC are common in the elderly population. Vitamin D insufficiency is simple to screen for and easy to treat with vitamin D supplementation. Checking serum 25-hydroxyvitamin D (calcidiol) is the best measure of vitamin D status. Maintaining serum concentrations of calcidiol above 80 nmol/L (32 ng/mL) may help prevent CRC. Daily calcidiol intake of 1000-2000 International Units (IU) can increase serum vitamin D to sufficient levels in most elderly persons, and based on available data may substantially lower the incidence of CRC with minimal risks.
Introduction
CRC is the third most commonly diagnosed cancer and the second most common cause of cancer death in the United States (1, 2). In a large prospective cohort study of healthy men, the most important factor for developing CRC was increasing age: the incidence of CRC is more than 50 times higher in subjects 60 to 79 years of age than in those younger than 40 years (2,3). Vitamin D insufficiency is strongly associated with increasing age and is widespread in the elderly (4-6). These and numerous other data to be reviewed in this paper provide strong scientific evidences that vitamin D insufficiency is a significant risk factor for CRC.
In this review we will begin with a discussion of the vitamin D status and CRC. Once that background information is provided, we will then review the clinical, in vitro, and in vivo animal data relevant to a preventive role of vitamin D on CRC. Studies evaluating the molecular mechanism of vitamin D’s inhibitory action on CRC are also briefly discussed. Based on the strength of overall evidence, we will follow up the sections mentioned above with a discussion of vitamin D metabolism and deficiency, how deficiency is determined, the amount of vitamin D required to correct it, and the safety profile of the
vitamin D. The goal of this review is to provide the reader with an arsenal of information to prevent vitamin D deficiency and thereby hopefully reduce the frequency of CRC.
Methods
A computerized English literature search of Medline since January 1962 to July 2008 was conducted by using predefined search terms. The search strategies for PubMed were “vitamin D” and “colon cancer”, “colorectal cancer”, or “cancer”. Bibliographies of all selected articles that included information on vitamin D and CRC were reviewed for other relevant articles.
The relationship between vitamin D status and CRC
Since the study by Garland and Garland in 1980 (7), the number of annual reports on the potential anticancer benefit from vitamin D has progressively increased (8-12). Tables 1, 2, and 3 provide a comprehensive list of all papers published to date on the relationship between vitamin D status and CRC. Figure 1 reveals a dose-response gradient for CRC according to serum calcidiol levels. Figure 2 represents the current concepts on anti-cancer mechanism of vitamin D. Figure 3 illustrates the current concepts of vitamin D metabolism.
There are several lines of evidence to support the anti-CRC benefit of vitamin D: 1. associations between solar ultraviolet-B (UV-B) radiation and CRC, 2. associations between serum vitamin D level and the incidence and/or mortality of CRC, and 3. associations between dietary and/or supplementary vitamin D and CRC or other cancers. 4. in vitro and in vivo animal studies, dealing with the potential of colon cancer protection by vitamin D.
Associations between solar UV-B radiation and CRC
Humans acquire most of their vitamin D (90-95%) through exposure to sunlight (3). Solar UV-B radiation is responsible for converting the precursor of vitamin D into previtamin D in the skin. Indices for effective sun exposure and skin vitamin D production include latitude, air-pollution, season, and skin pigmentation. Also, vitamin D synthesis through the skin is significantly lower in the elderly compared to the young (4).
Sixteen studies have evaluated the association between colon and/or rectal cancers and latitude, air-pollution, season, and skin cancer in the USA and other countries (5-20). Results from ecologic studies and meta-analysis studies are summarized in Table 1.
Out of seven studies in Table 1, five studies reported stastically significant associations between lower latitude, a surrogate of higher solar UV-B radiation, and decreased incidence and/or mortality of colon and/or rectal cancer (5, 7-10); two other studies in Table 1 reported strong trends in the same direction (18, 20). This work is consistent with the hypothesis that inadequate sun exposure is associated with increased risk for CRC.
One study examined the association between air-pollution, namely acid haze (UV-B blocking aerosols), and colon cancer mortality in 20 Canadian cities. A stastically significant positive association was found between air pollution and the age-adjusted mortality rate for colon cancer in both sexes (19).
Solar UV-B radiation exhibits a strong seasonal variation with the lowest level during the winter season. Four studies in Table 1 investigated the relationship between vitamin D induced from solar UV radiation with the prognosis of colon cancer (6, 11, 14, 16). In two studies, there were statistically significant superior prognoses in colon cancer death when the diagnosis was made in the summer, the season with the highest sun exposure, compared with the winter (6, 16). The third study revealed the same trend of higher colon cancer survival for patients diagnosed during the summer and autumn seasons without stastical significance (14). In the United Kingdom, the diagnosis of CRC in the summer was associated with improved survival compared with that in the winter (P < 0.01) (11).
Non-melanoma skin cancer (NMSC) is a potential surrogate for cumulative lifetime solar UV-B irradiation. Four studies in Table 1 evaluated the association between NMSC and subsequent development or mortality of colon and/or rectal cancer (12, 13, 15, 17). One study reported that NMSC was significantly associated with reduced CRC mortality in both sexes (13). Other studies reported that the risks for CRC were reduced, but the results were difficult to interpret (22, 25, 27).
There is a much higher incidence and mortality of CRC in African Americans than in Caucasians in the U. S. (21). With respect to this racial difference, there is indirect evidence that the higher incidence of CRC in African Americans is associated with a lower serum calcidiol value (22). Accordingly, in the same geographic regions in the U. S., African Americans have a serum calcidiol value of 19 ng/mL whereas Caucasians have a serum calcidiol value of 31 ng/mL (P < 0.01) (23). Because African Americans tend to have lower serum calcidiol values throughout the United States, it seems likely that the subjects with the higher incidence of CRC also expressed this difference in serum calcidiol values (24). Further work will be needed in order to explore this possibility. With respect to the cause of the lower serum calcidiol value in African Americans, it is of interest that in areas of chronic high sun exposure, African Americans are noted to have a statistically significantly lower calcidiol values compared to Caucasians (25). This raises the possibility that a lower level of serum calcidiol in African Americans is due to their skin pigmentation (26). The significance of this observation is that sun exposure is thought to be the major contributor to the serum calcidiol level in the U. S. (25, 27, 28). Another study has shown that low calcidiol level in African Americans is associated with higher risk for developing cancers of the digestive tract (29). The aforementioned studies support the conclusion that African Americans in the U. S. require relatively large amounts of vitamin D supplementation (30); and that such treatment could be a means to lower the incidence of CRC in African Americans.
Associations between serum vitamin D level and the incidence and/or mortality of CRC
Serum concentration of calcidiol is considered to be the best indicator of vitamin D status (108). It reflects vitamin D produced cutaneously and that obtained from food and/or supplements.
Fourteen studies evaluated the associations between serum vitamin D level and incidence and/or mortality of colon and/or rectal cancer (22, 31-43). Results from case-control, prospective cohort, and meta-analysis studies are summarized in Table 2.
Eight studies in Table 2 reported a stastical significant inverse association between calcidiol level and subsequent development of CRC (33, 35-40, 42), while the other two studies reported trends in the same direction that had borderline significance (31). A higher serum calcidiol level was associated with a decrease in the risk of CRC, and a lower calcidiol level was associated with an increase in the risk (12, 43, 45-49,51). A quantitative meta-analysis of five studies showed that individuals with ≥33 ng/ml (83 nmol/L) serum calcidiol had 50% lower incidence of CRC, compared individuals with ≤12 ng/ml serum calcidiol (49) (Fig 1). Two other studies showed a statistically significant association of serum calcidiol level with rectal cancer incidence, but not with colon cancer (22, 41). In both individual and meta-analysis studies, linear inverse relationships between vitamin D status and the risk of CRC have been found (36, 37, 40). Prospective, interventional studies are required in order to determine if this relationship is causal.
Other studies in Table 2 revealed a stastically significant association of serum vitamin D with mortality (43), survival (22), and staging of colon and/or rectal cancer (34). A statistically significant inverse relationship between CRC mortality and serum calcidiol level was noted (43). Among patients with CRC, higher serum calcidiol levels were associated with significant improvement in overall survival (P trend=0.02), and a trend toward improved CRC-specific mortality (52). An inverse correlation between serum 1,25-dihydroxyvitamin D (calcitriol) level and CRC stage has been demonstrated in CRC patients (34). From multivariable models, an increment of 25 nmol/L of calcidiol level was associated with a significant reduction in total cancer incidence and mortality and digestive-system cancer (including CRC) mortality (44).
One study reported no statistical significant association between serum calcidiol level and development of CRC (32). The potential problem with this study was that the serum calcidiol was sampled only once over the 10 to 17 year observational period (32, 41).
Associations between dietary and/or supplementary vitamin D and CRC or other cancers
There are very few naturally occurring dietary sources of vitamin D. The major fortified foods in the U. S. that contain vitamin D include milk (100 IU/8 oz), some orange juices (100 IU /8 oz), some breads, yogurts and cheeses. The content of vitamin D naturally found in food is highly variable. The flesh of fish (such as salmon, tuna, and mackerel) and fish liver oils are the best naturally occurring sources. Wild-caught salmon contains approximately 500 to 1000 IU of vitamin D3 in 3.5 oz, whereas farmed salmon in the US contains approximately 100 to 250 IU in 3.5 oz. When farmed salmon is baked, almost all of the vitamin D content is recovered from 3.5 oz of salmon. However, when the salmon is fried in vegetable oil, only 123 IU of vitamin D is recovered (45).
Twenty studies evaluated the associations between dietary and/or supplementary vitamin D and the incidence and mortality of colon and/or rectal cancer (Table 3) (36, 39, 46-63). One study evaluated the association between supplementary vitamin D and development of different cancers including CRC (64). All 21 studies, which includes case-control, prospective cohort, and meta-analysis studies, are summarized in Table 3.
Among twelve studies performed in the U.S. (36, 39, 46, 47, 50, 51, 53, 57, 60-63), five studies reported a stastically significant inverse association of dietary and/or supplementary vitamin D with incidence of colon and/or rectal cancer (36, 46, 47, 51, 61). Two other studies reported that higher total (dietary and supplementary) vitamin D intake was associated with significant reduction of CRC risk in men (60, 63). Four other studies reported trends in the same direction without stastical significance (50, 53, 57, 60). Two studies reported no significant inverse association between vitamin D intake and CRC incidence (39, 62).
Among eight studies done in Europe (48, 49, 52, 54-56, 58, 59), none showed a stastically significant association between vitamin D intake and both colon and rectal cancer development. Five studies in the Table 3 reported no association or trend between vitamin D intake and CRC risk (48, 54, 56, 58, 59). One other study reported a trend in the same direction without stastical significance (55). In another study, there was a significant inverse association between vitamin D intake and rectal cancers, not colon cancer (49). Another study showed significant inverse association between vitamin D and colon cancer, but not rectal cancer (52).
Lin et al suggested that the reasons why some dietary studies failed to observe the protective effect of vitamin D against the development of CRC might be inadequate measures of vitamin D intake, limited natural dietary sources of vitamin D, and the lack of information on sun exposure as an additional major source of vitamin D (62).
The most reliable scientific evidence is from randomized, double-blinded, placebo-controlled prospective studies. Two randomized, double blind, placebo-controlled trials involving postmenopausal women were conducted. The Women’s Health Initiative (WHI) trial using daily 400 IU of vitamin D and 1000 mg of calcium concluded that daily supplementation of vitamin D with calcium had no effect on the incidence of colorectal cancer (39). The other trial, by Lappe et al., utilizing 1100 IU vitamin D3 and 1400-1500 mg of calcium daily in the treatment group revealed that cancer incidence (including colon) was significantly lower in the women who were taking vitamin D and calcium than in the women taking placebo (P < 0.03) (64).
The principal difference between the trial of Lappe et al. and the WHI study is that Lappe et al. used a vitamin D dose sufficient to raise serum calcidiol by a biologically meaningful amount (from 71.8 ± 20.0 to 96 ± 21.4 nmol/L) (64), while the WHI study used a much lower dose of vitamin D (400 IU/d) and samples of women with substantially lower serum vitamin D levels (median serum calcidiol: 42 nmol/L) (39). In this regard, a meta-analysis of 16 studies demonstrated an increased calcidiol concentration of ≈1-2 nmol/L for each 100 IU per day of supplemental vitamin D (65). Four hundred IU/d of vitamin D supplementation would elevate serum calcidiol by only 4-8 nmol/L (from 42 to 46-50 nmol/l), which might not be adequate to protect against CRC. Vitamin D intake should be a sufficient amount to increase serum calcidiol level to at least normal in order to evaluate a relationship with CRC (40, 66).
In a systematic review of 18 studies, Gorham et al. concluded that there was an inverse dose-response relationship of oral intake of vitamin D with the risk of CRC. Intake of 1000 IU/d of vitamin D was associated with 50% lower incidence of CRC compared with <100 IU/d (P < 0.0001) (36).
Clinical Evidence of Other Effects of Vitamin D
There is now strong evidence that vitamin D has positive effects on several other health problems including type 1 DM, infection, stroke, myocardial infarction and overall mortality (14, (67)75-80).
Molecular Mechanisms of Anticancer Effect of Vitamin D
Over the past two decades, evidence has accumulated that vitamin D has anticancer properties through the vitamin D receptor (VDR) leading to a change in regulation of greater than 200 genes that have anti-proliferative (68-73), pro-differentiating (74-79), anti-angiogenetic (75), pro-apoptotic (80), and anti-metastatic effects in the colon (81-83) (Figure 2).
Vitamin D promotes the translocation of β-catenin from the nucleus to the plasma membrane and inhibits expression of β-catenin-TCF-4-target genes that is up regulated in most of human colon cancer where it affects the migratory, invasive and angiogenic phenotype of colon cancer cells (69,70) .
In an in vivo animal study vitamin D–sufficient mice had 40% smaller colonic tumors than vitamin D–deficient mice (82). The incidence of CRC induced by the carcinogens was significantly lowered after vitamin D supplementation in other in vivo animal studies (81, 84).
Vitamin D metabolism and treatment
The major sources of vitamin D for most humans are casual exposure of the skin to solar UV-B radiation and dietary intake (85). Vitamin D from the skin and diet is metabolized in the liver to calcidiol (86). The enzyme 1α-hydroxylase further metabolizes calcidiol to its active form calcitriol (87). Calcitriol also induces 24-hydroxylase expressions, which catabolizes calcitriol into biologically inactive calcitroic acid (88) (Figure 3).
It is important to note that the efficiency of production of vitamin D in the skin falls off substantially with aging (4, 89). Other factors that contribute to decreased sunlight exposure and vitamin D inadequacy include poor mobility and living in nursing homes (90, 91). Melanin skin pigmentation is an effective natural sunscreen, and darker skin pigmentation can greatly reduce UV-B–mediated cutaneous synthesis of vitamin D (73, 80, 92). Season and latitude affect vitamin D production in the skin. Winter season and high latitude decrease skin vitamin D synthesis (89).
The diagnosis of vitamin D insufficiency or sufficiency is made by a simple blood test, serum calcidiol analyses. An optimal value is considered to be at least 80 nmol/L (32 ng/mL) (36, 93, 94). The upper limit of normal serum calcidiol is 100 ng/mL (93). The serum calcidiol level between 32-100 ng/ml has not been well studied. Many elderly subjects will be found to require oral supplementation with vitamin D in order to achieve optimum levels in the blood. The recommended dose of vitamin D by Food and Nutritional Board in 1997 was 400-600 IU/day for elderly individuals (95). Unfortunately this level has been found to be ineffective in preventing colon cancer (45). Based on meta-analysis data Garland et al. suggested that a projected 50% reduction in the risk of CRC incidence would require an intake of 2000 IU/day of vitamin D (96). In some patients including African American more than 2000 IU per day will be required to achieve a serum level greater than 80 nmol/L (32ng/mL) (30).
Vitamin D causes hypercalcemia rarely when the "free" concentration of calcitriol is inappropriately high (97) . Calcidiol might cause hypercalcemia if taken with high amounts of calcium, as calcidiol increases intestinal calcium absorption . The National Academy of Science established that 2000 IU/day of vitamin D as the tolerable upper intake level which has no adverse effects (98). Available data on metabolic utilization of calcidiol indicate a total daily requirement of approximately 3000-5000 IU (99). Daily intake of 4000-10000 IU of calcidiol for 4-15 months did not increase serum calcium levels (95, 99, 100). When using higher doses of vitamin D (> 2000 IU per day), it is important to monitor serum calcium and calcidiol levels.
Summary and proposed future research studies
There is an urgent need for an inexpensive, readily available, safe, and effective modality for CRC prevention. Both CRC and vitamin D insufficiency are common in the elderly. The synthesis of vitamin D in the skin via exposure to solar UV-B radiation is decreased in the individuals who are elderly and with darker skin color. Vitamin D insufficiency is easy to screen for and treat with supplementation. There is a growing body of evidence that calcidiol has anti-cancer benefits and that a low calcidiol level is a risk factor for CRC development. This information supports the implementation of 1000 - 2000 IU of calcidiol daily to achieve a serum calcidiol level above 80 nmol/L (32 ng/mL) in elderly and particularly in high risk persons. The most urgent research needs is a prospective, interventional study which can define the dose and duration of vitamin D supplementation that would optimize its potential protective effects on the prevention of CRC. This study would need to include dark as well as white skinned individuals.
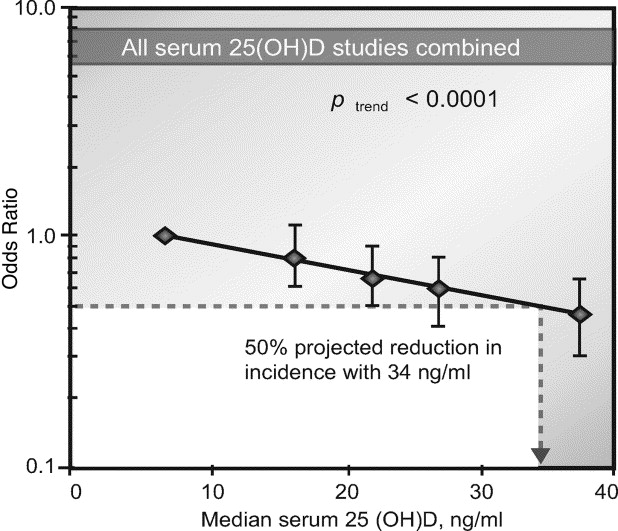
Figure 1. Dose–response gradient for colorectal cancer according to serum 25(OH)D concentration, all five studies combined (31-33, 35, 39) . The five points are the odds ratios for each quintile of 25(OH)D based on combined data from the five studies. Reprinted from reference (40), with permission.
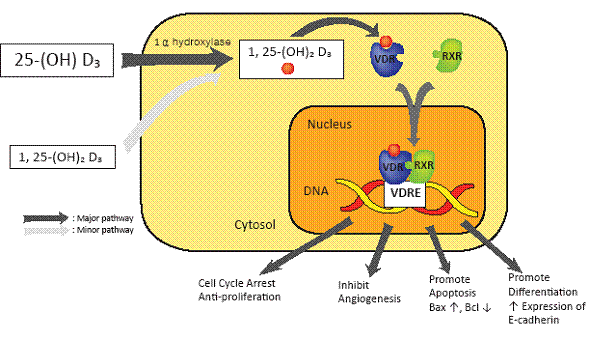
Figure 2. Overview of the Vitamin D Signaling Pathway. Calcidiol is converted by 1 α hydroxylase to calcitriol. Calcitriol binds to the vitamin D receptor (VDR) which dimerizes with the retinoid X receptor (RXR) to bind vitamin D response elements (VDRE) in target genes. VDRE activate target genes to inhibit proliferation, promote apoptosis, inhibit angiogenesis, promote differentiation, and increase expression of cell adhesion molecule E-cadherin.
Figure 3. Vitamin D Metabolism. Photochemical synthesis of vitamin D3 occurs cutaneously in response to ultraviolet B (sunlight) exposure. Vitamin D3 binds to vitamin D binding protein in the bloodstream, and is transported to the liver where calcidiol is produced by 25- hydroxylase. In kidney and other target tissues including intestinal cells, calcidiol is hydroxylated by 25-hydroxyvitamin D3- 1 α-hydroxylase to be an active vitamin D, calcitriol. Calcitriol have endocrine and paracrine/autocrine action. 24-hydroxylase catabolizes calcitriol into biologically inactive calcitroic acid.
Error! Objects cannot be created from editing field codes.
References
1. Espey DK, Wu XC, Swan J, et al. Annual report to the nation on the status of cancer, 1975-2004, featuring cancer in American Indians and Alaska Natives. Cancer 2007;110:2119-52.
2. www.cancer.org/downloads/STT/CAFF2005CR4PWSecured.pdf.
3. Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr 2004;79:362-71.
4. Holick MF, Matsuoka LY, Wortsman J. Age, vitamin D, and solar ultraviolet. Lancet 1989;2:1104-5.
5. Grant WB. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer 2002;94:1867-75.
6. Robsahm TE, Tretli S, Dahlback A, et al. Vitamin D3 from sunlight may improve the prognosis of breast-, colon- and prostate cancer (Norway). Cancer Causes Control 2004;15:149-58.
7. Freedman DM, Dosemeci M, McGlynn K. Sunlight and mortality from breast, ovarian, colon, prostate, and non-melanoma skin cancer: a composite death certificate based case-control study. Occup Environ Med 2002;59:257-62.
8. Mizoue T. Ecological study of solar radiation and cancer mortality in Japan. Health Phys 2004;87:532-8.
9. Spina C, Tangpricha V, Yao M, et al. Colon cancer and solar ultraviolet B radiation and prevention and treatment of colon cancer in mice with vitamin D and its Gemini analogs. J Steroid Biochem Mol Biol 2005;97:111-20.
10. Boscoe FP, Schymura MJ. Solar ultraviolet-B exposure and cancer incidence and mortality in the United States, 1993-2002. BMC Cancer 2006;6:264.
11. Lim HS, Roychoudhuri R, Peto J, et al. Cancer survival is dependent on season of diagnosis and sunlight exposure. Int J Cancer 2006;119:1530-6.
12. Grant WB. A meta-analysis of second cancers after a diagnosis of nonmelanoma skin cancer: additional evidence that solar ultraviolet-B irradiance reduces the risk of internal cancers. J Steroid Biochem Mol Biol 2007;103:668-74.
13. Grant WB. An ecologic study of cancer mortality rates in Spain with respect to indices of solar UVB irradiance and smoking. Int J Cancer 2007;120:1123-8.
14. Porojnicu A, Robsahm TE, Berg JP, et al. Season of diagnosis is a predictor of cancer survival. Sun-induced vitamin D may be involved: a possible role of sun-induced Vitamin D. J Steroid Biochem Mol Biol 2007;103:675-8.
15. Tuohimaa P, Pukkala E, Scelo G, et al. Does solar exposure, as indicated by the non-melanoma skin cancers, protect from solid cancers: vitamin D as a possible explanation. Eur J Cancer 2007;43:1701-12.
16. Moan J, Porojnicu A, Lagunova Z, et al. Colon cancer: prognosis for different latitudes, age groups and seasons in Norway. J Photochem Photobiol B 2007;89:148-55.
17. Soerjomataram I, Louwman WJ, Lemmens VE, et al. Are patients with skin cancer at lower risk of developing colorectal or breast cancer? Am J Epidemiol 2008;167:1421-9.
18. Garland CF, Garland FC. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol 1980;9:227-31.
19. Gorham ED, Garland CF, Garland FC. Acid haze air pollution and breast and colon cancer mortality in 20 Canadian cities. Can J Public Health 1989;80:96-100.
20. Emerson JC, Weiss NS. Colorectal cancer and solar radiation. Cancer Causes Control 1992;3:95-9.
21. Sharma S, O'Keefe SJ. Environmental influences on the high mortality from colorectal cancer in African Americans. Postgrad Med J 2007;83:583-9.
22. Ng K, Meyerhardt JA, Wu K, et al. Circulating 25-hydroxyvitamin d levels and survival in patients with colorectal cancer. J Clin Oncol 2008;26:2984-91.
23. Martins D, Wolf M, Pan D, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 2007;167:1159-65.
24. Bodnar LM, Simhan HN, Powers RW, et al. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr 2007;137:447-52.
25. Jacobs ET, Alberts DS, Foote JA, et al. Vitamin D insufficiency in southern Arizona. Am J Clin Nutr 2008;87:608-13.
26. Nesby-O'Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988-1994. Am J Clin Nutr 2002;76:187-92.
27. Holick MF. Vitamin D and sunlight: strategies for cancer prevention and other health benefits. Clin J Am Soc Nephrol 2008;3:1548-54.
28. Clemens TL, Adams JS, Henderson SL, et al. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet 1982;1:74-6.
29. Giovannucci E. Vitamin D and Cancer Incidence in the Harvard Cohorts. Ann Epidemiol 2008.
30. Talwar SA, Aloia JF, Pollack S, et al. Dose response to vitamin D supplementation among postmenopausal African American women. Am J Clin Nutr 2007;86:1657-62.
31. Garland CF, Comstock GW, Garland FC, et al. Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet 1989;2:1176-8.
32. Braun MM, Helzlsouer KJ, Hollis BW, et al. Colon cancer and serum vitamin D metabolite levels 10-17 years prior to diagnosis. Am J Epidemiol 1995;142:608-11.
33. Tangrea J, Helzlsouer K, Pietinen P, et al. Serum levels of vitamin D metabolites and the subsequent risk of colon and rectal cancer in Finnish men. Cancer Causes Control 1997;8:615-25.
34. Niv Y, Sperber AD, Figer A, et al. In colorectal carcinoma patients, serum vitamin D levels vary according to stage of the carcinoma. Cancer 1999;86:391-7.
35. Feskanich D, Ma J, Fuchs CS, et al. Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev 2004;13:1502-8.
36. Gorham ED, Garland CF, Garland FC, et al. Vitamin D and prevention of colorectal cancer. J Steroid Biochem Mol Biol 2005;97:179-94.
37. Sieg J, Sieg A, Dreyhaupt J, et al. Insufficient vitamin D supply as a possible co-factor in colorectal carcinogenesis. Anticancer Res 2006;26:2729-33.
38. Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst 2006;98:451-9.
39. Wactawski-Wende J, Kotchen JM, Anderson GL, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med 2006;354:684-96.
40. Gorham ED, Garland CF, Garland FC, et al. Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis. Am J Prev Med 2007;32:210-6.
41. Otani T, Iwasaki M, Sasazuki S, et al. Plasma vitamin D and risk of colorectal cancer: the Japan Public Health Center-Based Prospective Study. Br J Cancer 2007;97:446-51.
42. Wu K, Feskanich D, Fuchs CS, et al. A nested case control study of plasma 25-hydroxyvitamin D concentrations and risk of colorectal cancer. J Natl Cancer Inst 2007;99:1120-9.
43. Freedman DM, Looker AC, Chang SC, et al. Prospective study of serum vitamin D and cancer mortality in the United States. J Natl Cancer Inst 2007;99:1594-602.
44. Giovannucci E. Strengths and limitations of current epidemiologic studies: vitamin D as a modifier of colon and prostate cancer risk. Nutr Rev 2007;65:S77-9.
45. Lu Z, Chen TC, Zhang A, et al. An evaluation of the vitamin D3 content in fish: Is the vitamin D content adequate to satisfy the dietary requirement for vitamin D? J Steroid Biochem Mol Biol 2007;103:642-4.
46. Garland C, Shekelle RB, Barrett-Connor E, et al. Dietary vitamin D and calcium and risk of colorectal cancer: a 19-year prospective study in men. Lancet 1985;1:307-9.
47. Bostick RM, Potter JD, Sellers TA, et al. Relation of calcium, vitamin D, and dairy food intake to incidence of colon cancer among older women. The Iowa Women's Health Study. Am J Epidemiol 1993;137:1302-17.
48. Ferraroni M, La Vecchia C, D'Avanzo B, et al. Selected micronutrient intake and the risk of colorectal cancer. Br J Cancer 1994;70:1150-5.
49. Pritchard RS, Baron JA, Gerhardsson de Verdier M. Dietary calcium, vitamin D, and the risk of colorectal cancer in Stockholm, Sweden. Cancer Epidemiol Biomarkers Prev 1996;5:897-900.
50. Martinez ME, Giovannucci EL, Colditz GA, et al. Calcium, vitamin D, and the occurrence of colorectal cancer among women. J Natl Cancer Inst 1996;88:1375-82.
51. Kearney J, Giovannucci E, Rimm EB, et al. Calcium, vitamin D, and dairy foods and the occurrence of colon cancer in men. Am J Epidemiol 1996;143:907-17.
52. La Vecchia C, Braga C, Negri E, et al. Intake of selected micronutrients and risk of colorectal cancer. Int J Cancer 1997;73:525-30.
53. Marcus PM, Newcomb PA. The association of calcium and vitamin D, and colon and rectal cancer in Wisconsin women. Int J Epidemiol 1998;27:788-93.
54. Pietinen P, Malila N, Virtanen M, et al. Diet and risk of colorectal cancer in a cohort of Finnish men. Cancer Causes Control 1999;10:387-96.
55. Dalberg J, Jacobsen O, Nielsen NH, et al. Colorectal cancer in the Faroe Islands--a setting for the study of the role of diet. J Epidemiol Biostat 1999;4:31-6.
56. Levi F, Pasche C, Lucchini F, et al. Selected micronutrients and colorectal cancer. a case-control study from the canton of Vaud, Switzerland. Eur J Cancer 2000;36:2115-9.
57. Kampman E, Slattery ML, Caan B, et al. Calcium, vitamin D, sunshine exposure, dairy products and colon cancer risk (United States). Cancer Causes Control 2000;11:459-66.
58. Jarvinen R, Knekt P, Hakulinen T, et al. Prospective study on milk products, calcium and cancers of the colon and rectum. Eur J Clin Nutr 2001;55:1000-7.
59. Terry P, Baron JA, Bergkvist L, et al. Dietary calcium and vitamin D intake and risk of colorectal cancer: a prospective cohort study in women. Nutr Cancer 2002;43:39-46.
60. McCullough ML, Robertson AS, Rodriguez C, et al. Calcium, vitamin D, dairy products, and risk of colorectal cancer in the Cancer Prevention Study II Nutrition Cohort (United States). Cancer Causes Control 2003;14:1-12.
61. Slattery ML, Neuhausen SL, Hoffman M, et al. Dietary calcium, vitamin D, VDR genotypes and colorectal cancer. Int J Cancer 2004;111:750-6.
62. Lin J, Zhang SM, Cook NR, et al. Intakes of calcium and vitamin D and risk of colorectal cancer in women. Am J Epidemiol 2005;161:755-64.
63. Park SY, Murphy SP, Wilkens LR, et al. Calcium and vitamin D intake and risk of colorectal cancer: the Multiethnic Cohort Study. Am J Epidemiol 2007;165:784-93.
64. Lappe JM, Travers-Gustafson D, Davies KM, et al. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr 2007;85:1586-91.
65. Cranney A, Weiler HA, O'Donnell S, et al. Summary of evidence-based review on vitamin D efficacy and safety in relation to bone health. Am J Clin Nutr 2008;88:513S-519S.
66. Bischoff-Ferrari HA, Giovannucci E, Willett WC, et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 2006;84:18-28.
67. Mohr SB, Garland CF, Gorham ED, et al. The association between ultraviolet B irradiance, vitamin D status and incidence rates of type 1 diabetes in 51 regions worldwide. Diabetologia 2008;51:1391-8.
68. Bettoun DJ, Buck DW, 2nd, Lu J, et al. A vitamin D receptor-Ser/Thr phosphatase-p70 S6 kinase complex and modulation of its enzymatic activities by the ligand. J Biol Chem 2002;277:24847-50.
69. Scaglione-Sewell BA, Bissonnette M, Skarosi S, et al. A vitamin D3 analog induces a G1-phase arrest in CaCo-2 cells by inhibiting cdk2 and cdk6: roles of cyclin E, p21Waf1, and p27Kip1. Endocrinology 2000;141:3931-9.
70. Wali RK, Khare S, Tretiakova M, et al. Ursodeoxycholic acid and F(6)-D(3) inhibit aberrant crypt proliferation in the rat azoxymethane model of colon cancer: roles of cyclin D1 and E-cadherin. Cancer Epidemiol Biomarkers Prev 2002;11:1653-62.
71. Yang W, Bancroft L, Nicholas C, et al. Targeted inactivation of p27kip1 is sufficient for large and small intestinal tumorigenesis in the mouse, which can be augmented by a Western-style high-risk diet. Cancer Res 2003;63:4990-6.
72. Watson AJ. An overview of apoptosis and the prevention of colorectal cancer. Crit Rev Oncol Hematol 2006;57:107-21.
73. Chen A, Davis BH, Sitrin MD, et al. Transforming growth factor-beta 1 signaling contributes to Caco-2 cell growth inhibition induced by 1,25(OH)(2)D(3). Am J Physiol Gastrointest Liver Physiol 2002;283:G864-74.
74. Shah S, Islam MN, Dakshanamurthy S, et al. The molecular basis of vitamin D receptor and beta-catenin crossregulation. Mol Cell 2006;21:799-809.
75. Chung I, Karpf AR, Muindi JR, et al. Epigenetic silencing of CYP24 in tumor-derived endothelial cells contributes to selective growth inhibition by calcitriol. J Biol Chem 2007;282:8704-14.
76. Larriba MJ, Valle N, Palmer HG, et al. The inhibition of Wnt/beta-catenin signalling by 1alpha,25-dihydroxyvitamin D3 is abrogated by Snail1 in human colon cancer cells. Endocr Relat Cancer 2007;14:141-51.
77. Aguilera O, Pena C, Garcia JM, et al. The Wnt antagonist DICKKOPF-1 gene is induced by 1alpha,25-dihydroxyvitamin D3 associated to the differentiation of human colon cancer cells. Carcinogenesis 2007;28:1877-84.
78. Palmer HG, Gonzalez-Sancho JM, Espada J, et al. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol 2001;154:369-87.
79. Wilson AJ, Velcich A, Arango D, et al. Novel detection and differential utilization of a c-myc transcriptional block in colon cancer chemoprevention. Cancer Res 2002;62:6006-10.
80. Diaz GD, Paraskeva C, Thomas MG, et al. Apoptosis is induced by the active metabolite of vitamin D3 and its analogue EB1089 in colorectal adenoma and carcinoma cells: possible implications for prevention and therapy. Cancer Res 2000;60:2304-12.
81. Cross HS, Lipkin M, Kallay E. Nutrients regulate the colonic vitamin D system in mice: relevance for human colon malignancy. J Nutr 2006;136:561-4.
82. Harris DM, Go VL. Vitamin D and colon carcinogenesis. J Nutr 2004;134:3463S-3471S.
83. Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer 2007;7:684-700.
84. Fichera A, Little N, Dougherty U, et al. A vitamin D analogue inhibits colonic carcinogenesis in the AOM/DSS model. J Surg Res 2007;142:239-45.
85. Chen TC, Chimeh F, Lu Z, et al. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys 2007;460:213-7.
86. DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 2004;80:1689S-96S.
87. Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266-81.
88. Kallay E, Bises G, Bajna E, et al. Colon-specific regulation of vitamin D hydroxylases--a possible approach for tumor prevention. Carcinogenesis 2005;26:1581-9.
89. Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab 1988;67:373-8.
90. Johnson MA, Kimlin MG. Vitamin D, aging, and the 2005 Dietary Guidelines for Americans. Nutr Rev 2006;64:410-21.
91. Bouuaert C, Vanmeerbeek M, Burette P, et al. [Vitamin D deficiency in elderly men living in urban areas, at home or in institutions.]. Presse Med 2008;37:191-200.
92. Armas LA, Dowell S, Akhter M, et al. Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color. J Am Acad Dermatol 2007;57:588-93.
93. Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr 2005;135:317-22.
94. Cross HS, Bises G, Lechner D, et al. The Vitamin D endocrine system of the gut--its possible role in colorectal cancer prevention. J Steroid Biochem Mol Biol 2005;97:121-8.
95. Hathcock JN, Shao A, Vieth R, et al. Risk assessment for vitamin D. Am J Clin Nutr 2007;85:6-18.
96. Garland CF, Grant WB, Mohr SB, et al. What is the dose-response relationship between vitamin D and cancer risk? Nutr Rev 2007;65:S91-5.
97. Vieth R. Vitamin D toxicity, policy, and science. J Bone Miner Res 2007;22 Suppl 2:V64-8.
98. Yates AA, Schlicker SA, Suitor CW. Dietary Reference Intakes: the new basis for recommendations for calcium and related nutrients, B vitamins, and choline. J Am Diet Assoc 1998;98:699-706.
99. Heaney RP, Davies KM, Chen TC, et al. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 2003;77:204-10.
100. Vieth R, Kimball S, Hu A, et al. Randomized comparison of the effects of the vitamin D3 adequate intake versus 100 mcg (4000 IU) per day on biochemical responses and the wellbeing of patients. Nutr J 2004;3:8.
Conflict of Interest
Guarantor of the article: Michael J Walter, M.D., F.A.C.P., F.A.C.G.
Specific author contributions: Dae S Rheem: library search, and drafting the manuscript. David J Baylink: suggested, outlined, and edited the manuscript. Snorri Olafsson: edition and statistics of the manuscript. Christian Jackson: racial disparity in the manuscript. Michael H. Walter: suggested, outlined, and edited the manuscript.
Financial support: None.
Potential competing interests: None
Table 1. Summary of Studies on Association between Solar UV-B Light with Colon and/or Rectal Cancer.
Authors, Year, Country, References |
Study Design |
Association with
Vitamin D |
Stastical Significance |
Measure of Association |
Garland and Garland,
USA, 1980, (7) |
Ecologic |
CCA, Mortality |
Significant inverse association |
Pearson product-moment correlation = - 0.9. |
Gorham et al., 1989, Canada, (19). |
Ecologic |
CCA,
Mortality |
Significant positive association |
Women(r = +.74, P = 0.003) and men ( r = +.61, P = 0.03). |
Emerson and Weiss, USA, 1992, (20). |
Ecologic |
CRC,
Incidence |
No statistical data available. |
Positive trend |
Grant,
2002, USA, (5) |
Ecologic |
CRC,
Incidence |
Stastically significant. |
RC, -0.62 to -0.69, P < 0.001 |
Freedman et al., 2002, USA, (7) |
Case control |
CCA., Mortality |
Significantly and negatively associated |
OR= 0.90 (95% CI= 0.88 - 0.92). |
Mizoue, 2004, Japan, (8). |
Ecologic |
CRC, Mortality |
Significant inverse association |
(CC, -0.47to -0.54, P <0.01). |
Robsahm et al., 2004, Norway, (6). |
Ecologic |
CCA,,
Mortality,
Season |
Stastically significant. |
RR=0.82, 95% CI= 0.77-087, in women
RR=0.84, 95% CI=0.79-0.90 in men. |
Spina et al., 2005, USA, (19) |
Ecologic |
CRC,
Incidence |
Stastically significant. |
PC=0.44 (P < 0.005). |
Boscoe et al., 2006, USA, (10). |
Ecologic |
CRC, Incidence, Mortality |
Significant inverse relationship |
RR (95% CI): CCA. In; M, 1.11 (1.08-1.13), F, 1.14 (1.11-1.16), Mo; M, 1.27 (1.24-1.30), F, 1.24 (1.22-1.27). RCA. In; M, 1.27 (1.23-1.32), F, 1.14 (1.09-1.18), Mo; M, 1.53 (1.45-1.60), F, 1.37 (1.30-1.44). |
Lim et al., 2006, U.K., (11). |
Ecologic |
CRC,
Survival |
Stastically significant in female |
In female (P < 0.01), but not in male. |
Grant, 2007, Spain, (13). |
Ecologic |
NMSC & CRC,
Mortality |
Significant inverse correlation |
P < 0.01. |
Grant, 2007,
Eight counties, (12). |
Meta-analysis |
SCC, CRC, Incidence |
Stastically significant for SCC, marginally insignificant for NMSC |
SCC vs CCA (RR: 0.78 (0.60–0.96), RCA (RR: 0.65 (0.31–0.99).
NMSC vs CCA (RR:0.87 (0.73–1.02), RCA (RR: 0.83 (0.66–0.99) |
Porojnicu et al., 2007, Norway, (14). |
Ecologic |
CRC, Season, Prognosis |
No statistical data available. |
A seasonal variation of prognosis of CCA. |
Tuohimaa et al., 2007, Thirteen countries, (15). |
Ecologic |
NMSC, CRC,
Risk |
Significantly lower in sunny countries. |
After basal cell ca. SIR(S)/SIR(L)=0.65 (95%CI=0.58-0.72); after non-basal cell ca. SIR(S)/SIR(L)=0.58 (95%CI=0.50-0.67). |
Moan et al., 2007, Norway, (16). |
Ecologic |
CRC,
Season, Survival |
No statistical data available. |
The survival of CCA, is dependent on the season of the diagnosis. |
Soerjomataram et al., 2008, Netherlands, (17). |
Ecologic |
NMSC, CRC,
Risk |
Stastically significant. |
NMSC: M (SIR=0.64, 95% CI: 0.71, 0.97); SCC (SIR=0.64, 95% CI: 0.43, 0.93); |
Abbreviations: PC: Pearson product-moment correlation; SCC: squamous cell carcinomas; BCC: basal cell carcinomas; CI: confidence interval; SIR: standardized incidence ratio; CRC: colorectal cancer; OR: odd ratio; RR: relative risk; RC: regression coefficiency; CC: correlation coefficiency; M: males; F: female; NMSC: non-melanoma skin cancer. In: incidence; Mo: mortality; CCA; Colon cancer; RCA; Rectal cancer
Table 2. Summary of Studies on Associations between Serum Vitamin D Level and Colon, and/or Rectal Cancer.
Authors, Year, Country, References |
Study Design |
Association with Vitamin D |
Stastical Significance |
Measure of Association |
Garland et al., 1989,
USA (31). |
Case Control |
Colon ca.
Risk. |
Borderline significant association |
P trend = 0.05 |
Braun et al., 1995,
USA (32). |
Case Control |
Colon ca.
Risk |
No statistical significance |
|
Tangrea et al., 1997,
Finland (33). |
Case Control |
CRC,
Risk. |
Stastically significant. |
P < 0.01 |
Niv et al., 1999,
Israel (34). |
Case Control |
CRC,
Stage. |
Significant inverse correlation |
P < 0.05 |
Feskanich et al., 2004, USA (35). |
Case Control |
CRC,
Risk. |
Significant inverse linear association |
P = 0.02 |
Gorham et al., 2005, (36). |
Meta Analysis |
CRC,
Incidence. |
Stastically significant. |
P < 0.01 |
Sieg et al., 2006, Germany (37). |
Prospective,
Cohort |
CRC,
Risk. |
Stastically significant. |
P < 0.001 |
Giovannucci et al., 2006, USA (38). |
Prospective
Cohort |
Digestive-system ca.,
Mortality. |
Stastically significant. |
RR=0.55, 95%CI= 0.41 to 0.74 |
Wactawski-Wende et al., 2006, USA (39). |
Case Control |
CRC, Risk |
Stastically significant. |
P trend = 0.02 |
Gorham et al., 2007, USA (40). |
Meta Analysis |
CRC, Risk |
Stastically significant. |
P trend = 0.0001 |
Otani et al., 2007,
Japan (41). |
Case Control |
Rectal ca, Risk |
Stastically significant. |
P trend = 0.006 in men,
P trend = 0.04 in women |
Wu et al., 2007,
USA (42). |
Case Control |
Colon ca., Risk |
Stastically significant. |
OR=0.46, 95% CI= 0.24 -0.89; - P trend = 0.0005 |
Freedman et al., 2007, USA (43). |
Prospective,
Cohort |
CRC, Mortality |
Significant inverse relationship |
P trend = 0.02 |
Ng et al., 2008,
USA (22). |
Prospective,
Cohort |
CRC, Survival |
Stastically significant. |
P trend = 0.02 |
Abbreviations: RR: relative risk; OR: odds ratio; HR: hazard ratio; CI: confidence interval; CRC: colorectal cancer; HR: hazard ratio.
Table 3. Summary of Studies on Associations between Dietary and/or Supplementary Vitamin D and Colon, Rectal, and/or other Cancer.
Authors, Year, Country, References |
Study Design |
Association with Vitamin D Sex |
Stastical Significance |
Measure of Association |
Garland et al., 1985,
USA (46). |
Prospective cohort |
CRC,
Risk
Both |
Significant inverse correlation |
(RR = 0.32, P trend < 0.05). |
Bostick et al., 1993, USA (47). |
Prospective cohort |
Colon,
Women |
Significant inverse association |
RRs 0.54 (95% CI = 0.35-0.84) |
Ferraroni et al., 1994,
Italy (48). |
Case control |
CRC,
Both |
No statistical significance |
|
Pritchard et al., 1996
Sweden (49). |
Case control |
CRC,
Both |
Significant in rectal Ca, borderline significance in colon Ca |
Rectal Ca.(OR, 0.5; 95% CI, 0.3-0.9) . Colon Ca. (OR = 0.6; 95% CI= 0.4-1.0). |
Martinez et al., 1996, USA (50). |
Prospective cohort |
CRC,
Risk
Female |
Significant inverse association |
RR: 0.42 (95% CI = 0.19-0.91) for total vitamin D. |
Kearney et al., 1996, USA (51). |
Prospective cohort |
Colon,
Occurrence,
Male |
Significant inverse association |
RR 0.54 (95 % CI 0.34-0.85, P = 0.0006). |
La Vecchia et al., 1997, Italy (52). |
Case control |
CRC,
Both |
The association was significant for colon ca., but not for rectal ca. |
Colon ca. [OR = 0.81; 95% CI, 0.7-0.9], Rectal ca. (OR = 1.03; 95% CI, 0.9-1.2). |
Marcus et al., 1998,
USA (50). |
Case control |
CRC,
Female |
Stastically insignificant, but trend +. |
Colon ca.: OR = 0.7, 95% CI: 0.4-1.1, rectal ca.: OR = 0.8, 95% CI: 0.5-1.5). |
Pietinen et al., 1999, Finland (54). |
Case control |
CRC,
Risk,
Male |
No significant association |
|
Dalberg et al., 1999, Denmark,(55). |
Case control |
CRC, Both |
No significant association |
|
Levi et al., 2000,Switzland (56). |
Case control |
CRC,
Both |
No significant association |
|
Kampman et al., 2000, USA, (57). |
Case control |
CRC,
Risk,
Both |
Stastically insignificant, but trend +. |
Men (OR = 0.5, 95% CI: 0.2-1.1), Women: OR = 0.6, 95% CI: 0.4-1.1). |
Jarvinen et al., 2001, Finland (58). |
Prospective cohort |
CRC, Both |
Not significantly related |
|
Terry et al., 2002, Sweden (59). |
Prospective cohort |
CRC,
Risk,
Women |
Not significantly related |
|
McCullough et al., 2003, USA (60). |
Prospective cohort |
CRC,
Risk,
Both |
Significant inverse association only in men. |
RR = 0.71, 95% CI 0.51-0.98, P trend = 0.02). |
Slattery et al., 2004,USA (61). |
Case control |
CRC,
Both |
Significant inverse association only in women, |
OR = 0.52; 95% CI = 0.32-0.85. |
Gorham et al., 2005, USA (36). |
Meta analyst |
CRC,
Incidence
Both |
Significant only individuals taking ≥ 1000 IU/day vitamin D |
P<0.0001. |
Lin et al., 2005, USA (62). |
Prospective cohort |
CRC, Women |
No significant association |
RR = 1.34; 95% CI: 0.84, 2.13; P trend=0.08. |
Wactawski-Wende et al.,
2006, USA (39). |
Case control |
CRC,
Risk,
Women |
No significant association |
HR, 1.08; 95 % CI, 0.86 to 1.34; P = 0.51. |
Park et al., 2007, USA (63). |
Prospective cohort |
CRC,
Risk,
Both |
Borderline significant association in men |
RR = 0.72, 95% CI: 0.51, 1.00; P trend = 0.03 |
Lappe et al., 2007, USA (64). |
RCT. |
Non-Skin cancer,
Incidence,
Women |
Significant reduction of cancer incidence |
RR: 0.402 (95% CI: 0.20, 0.82; P < 0.013. |
Abbreviations: RR: relative risk; OR: odds ratio; HR: hazard ratio; CI: confidence interval; CRC: colorectal cancer; HR: hazard ratio. RCT: double blinded randomized placebo-controlled trial
Biography of the Author
After postdoctoral fellowships at Harvard Medical School and the Postgraduate Medical School of London, Hammersmith Hospital, in 1966 Dr. Baylink moved to the University of Washington in Seattle, Washington, where he subsequently rose to Professor of Medicine in 1977. In 1981, Dr. Baylink moved to Loma Linda University, where he is currently Distinguished Professor of Medicine and Professor of Biochemistry, Orthopedic Surgery and Dentistry, Director of the Molecular Regeneration Laboratory and Director of the osteoporosis clinic. At the Jerry L. Pettis Veterans Administration Medical Center he was the Associate Vice-President for Medical Affairs for Research, as well as Director, Musculoskeletal Disease Center for 5 years ending in 2005.
Dr. Baylink has received several awards for his work on bone and mineral metabolism, including the Medical Investigator Award from the Veterans Administration, and the Uehlinger Medal in 2005 (he is the only non-European to receive this honor). In 2006, became an Honorary Member Austrian Society for Bone and Mineral Research. In 2007 received the national RIB Award. In 2008, he received the William F. Neumann Award. This prestigious award is the highest honor given by The American Society of Bone and Mineral Research, a scientific Society with over 6000 members.
Dr. Baylink has published well over 600 papers in the musculoskeletal field, including papers in molecular genetics, tissue regeneration, stem cell therapy and gene therapy.
Dr. Baylink's hobbies include tennis, bicycling, motorcycle riding, skiing, creative writing, and electronic gadgets.
Dr. Baylink and his wife, Colleen, have 2 children and six grandchildren. Mrs. Baylink is a retired schoolteacher.
|

